Financing of start-ups in the pharmaceutical biotechnologies
Academic Advice Note issued by the National Academy of Technologies of France (NATF)
The financial chain for pharmaceutical biotech start-ups in France has broken down because of a lack of funding sources, mainly for the downstream phases, which dissuades upstream investors. This trend is accompanied by a decrease in the will of all actors in the field to assume risks (for both conjunctural and structural reasons).
If we wish to re-introduce a positive leverage, for both creation and starting phases and also in development projects, there must be a simplification and better coordination in public policies, plus a review of certain fiscal measures and plans to envisage new measures (life insurances, for example).
The controlling/management mechanisms for public funding (subsidies, financial aid, reimbursable loans, SATTs (companies to speed up technological transfer), seed funding, OSEO) should allow access for private actors (venture capital investors, industry at large, other start-ups, representative associations).
The National Academy of Technologies of France (NATF) can offer the expertise of its Fellows to help the public authorities to identify the most efficient solutions and can advise as how to implement them.
Over the past decade, pharmaceutical innovation has been located for some 60% in the USA, 30-35% in Europe and more recently in Asia (20% in 2009). Out of 252 new medicinal drugs authorised by the FDA (Federal Drug Administration) between 1998 and 2007, one half came from biotech companies and university laboratories. Over the same period, it could be observed that the biotechnological health sector went through several phases, ranging from enthusiasm in the decade 1990-2000 to the difficulties encountered in the financial crisis 2008-2009. Difficulties vary from country to country, but it is true to say that the financial crisis was particularly serious in Europe and in France.
The dual aim of this Academic Advice Note is 1° to explain why the “virtuous circle” of the 1990s (that saw creation, seed funding, venture capital fund raising, registration in stock exchanges) no longer works and 2° to propose solutions that would at least improve on the current situation. The sector concerned is mainly that of pharmaceutical start-ups because of their importance, on one hand, and the financial difficulties that latter have met, especially because the lead times and development costs have considerably increased as concomitantly their needs for funds.
Biotechnological health sector start-ups
Biotechnological health sector start-ups can be grouped into one of three categories:
- Companies devoted to development of therapies, techniques for diagnosis and vaccines (biotechnological pharmaceutical start-ups). Their primary characteristic is the lead time needed for development of products, often extending beyond 10 years with very high investments. Needs for venture capital funding here lie between 30 and 80 M€ and in certain cases in excess of 150 M€. The principal problem here is to secure the financial cover needed;
- Medical technology intensive companies, providing “from scalpels to scanners” (medical imaging, hospital equipment, prostheses). They call for lower levels of financial cover , can be developed in an autonomous manner and can produce a positive financial return in just a few years. This is an innovative sector that is rapidly evolving: it is a very active sector in Germany with positive budget balance figures whilst the French counterparts register a 2.5 billion€ deficit;
- The pharmaceutical, biotechnological (and other) industry service sector. These companies need less finance but they are not easy to find, given the limited prospects for expansions (more business, higher trade turnover figures).
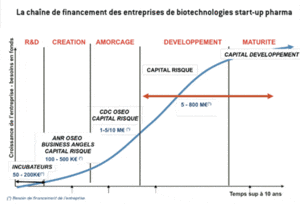
In order to succeed, the creation of a start-up needs a reliable scientific base, a clear intellectual property standing, a skilled and motivated scientific team of co-workers, an excellent “business” oriented management and a coherent group of investors to back them. The various phases through which a start-up can evolve are displayed in the following diagram (source: adapted from CDC Enterprise):
The ‘normal’ progress path shown here no longer operates – for structural reasons – because venture capital funds are no longer alone in a position to correctly finance the seed funding and development phases. This situation has led certain start-ups to fail and exit the scene while others choose to concentrate their R&D efforts and to seek (without a great deal of success upstream of development) help and collaboration from and with major pharmaceutical groups capable of assuming the final phases of product development to the extent that the latter themselves have evolved. Because of merger/acquisition processes among the major companies here, some have simply abandoned certain lines of research, fired personnel and thereby have externalised a large amount of their research (30 to 40%). This is why the pharmaceutical start-ups accept, at least partly, the costs of the upstream R&D phases, hence the strategic importance of securing financial funding for these activities.
The French system for financing biotechnological start-ups
The French system associated with financing of biotechnological pharmaceutical start-ups is not only legion but at the same time patchy and difficult to understand:
- There are multiple sources for public financing of start-ups: a national competitive process for the creation of innovating enterprises, followed by OSEO financial aids, nurseries (advisory services and premises) and (in a marginal manner) aid from Business Angels. Most of these financial aids are granted with the proviso that the applicants demonstrate they own funds, this being an especially reasonable approach. Access to and availability of own funds is therefore primordial;
- For seed money, CDC Enterprises invests in national, regional and sectorial funds. The BIOAM Fund, managed by CDC Enterprises and specific to the field of biotechnologies, was obliged to act as a conventional venture capitalist because of its particular profitability constraints;
- The further downstream, public, part related to financing of enterprise is assured by OSEO and in particular by OSEO ISI;
- Several measures connected with the status and fiscal situation of recently founded enterprises have been implemented: the status of secunded research scientists, the law on innovation that authorises public scientist to create a start-up to value add to their research work and findings, statutes for recently founded innovative companies, the research tax rebate system, the joint funds for innovation investments (FCPI), reduced taxes on personal fortunes if the money is invested in SMEs that are not registered with the stock exchange.
The national loan [Treasury bonds issued by the French Government] will largely change the odds: via an investment of 1.55 billion euros aimed at enabling the emergence of a bio-economic sector based on most recent discoveries in life sciences and on new forms of valorisation of renewable biological resources; investments of 0.85 billion euros to finance teaching hospital Institutes (IHUs), optimisation of technology transfer through the creation of special companies for the acceleration of transfer actions (SATTs). Moreover, in the framework of the national loan, a seed money fund (with 400 M€) has been set up. Specific measures in favour of biotechnologies must be enacted so that the future of financial finding of pharmaceutical can be assured.
The most obvious lack of funding lies in the seeding phase, which runs from scientific validation of research work (lab. results) to ‘proof of concept’ (phase I/II in clinical studies). The average cost of this phase is approx. 10 M€ over 3 to 4 years but can climb to 20 M€, with an unknown degree of probable success, that can no longer be financed by venture capital (or with lack of will to do so) given that there is a very high risk of losing the outlay which is not compensated by gains in ‘good’ operations.
Problems specific to seeding stages and to development of start-ups
Since the early 1980s, predominantly in the USA, the reigning economic model was the financing of start-ups through venture capital funds, which themselves were financed by long (and even very long term) term investments. This system made available huge amounts of money and led to a series of success stories like, for example, Amgen, Genentech, Applied Biosystems, Centocor ou Genzyme. France did not have very long term capital resources and in fact ran into considerable difficulty when endeavouring to implement resources of a sufficiently high level to ensure success. Despite this handicap, France was able to fund between 250 and 300 companies, some of which were able to raise several tens of millions of euros. Today, the prevailing venture capital model no longer operates satisfactorily and the currency markets prove less efficient for those companies that have no turnover figures to show. It can clearly be deduced that correct funding of pharmaceutical start-ups is no longer possible in France.
And there is no possible alternative: in year 2009, the differences in investment level (for a given spread of activities) in biotechnical start-ups between the USA (12.5 billion euros) and Europe (2.2 billion euros) are huge; not one venture capital fund, or venture investment fund (FCPR) investing in the field of biotechnologies has been able to raise any money since 2009!
Of course, public authorities did create the strategic investment fund, designed to procure investment for companies that already exist; likewise the INNOVIO Fund managed by CDC Entreprises (139 M€) to finance recently registered companies and France Investissement (900 M€ from various national, regional investment fund processes (and other sectors affiliated to CDC Entreprises).
France occupies an unusual position inasmuch as there are funds such as the FCPI – that have been extended to end 2012 – but with a constraint limiting the financial outlay possible to 2.5 M€/yr/company and this is not very well adapted to the situations arising in biotechnological sectors – and the ISF/SME measure that is used to raise money from private individual through a “fortune related” tax incentive. FCPI and ISF/SME together have played a considerable role, notably in re-financing companies that had raised earlier funding from conventional venture capital sources, but they cannot alone be seen as satisfactory substitutes.
The level of involvement of major industrial groups in the financing of start-ups is limited as yet, even if we can observe recent creation of ‘corporate’ investment funds. France did not make the most of “cluster” policies where the proximity fcator of industrial leaders and young innovating companies can lead to implementation of financial and managerial support circuits that help accelerate their development.
Finally what we must accept is that there is no major technological development fund in France, one that would enable autonomous development for start-ups.